Innovations in Carbon Capture
Neil Williams started his college career as a biology student with hopes of going to medical school. However, all that changed when he took organic chemistry.
“I fell in love with the research and became one of those students who was in the lab all the time,” says Williams, a PhD student in the Department of Chemistry.
His research involved binding different metals in water using a variety of different compounds. This experience provided Williams with the fundamental tools necessary to be part of a team at the Department of Energy’s Oak Ridge National Laboratory that recently discovered a simple, reliable process for capturing carbon dioxide from the air, which offers a new option for carbon capture and storage strategies to combat global warming.
“We stumbled on the process completely by chance,” Williams says. “Our initial project was to develop a way to remove sulfate from seawater.” Offshore oil rigs use seawater to cool the pipes they use to pump oil, but the sulfate in seawater reacts with the barium in the pipes and builds up over time, similar to the way cholesterol builds up in the arties of a human body. Instead of putting the pipes on a diet, however, the oil companies simply replace them, which costs money, time, and has a toll on the environment.
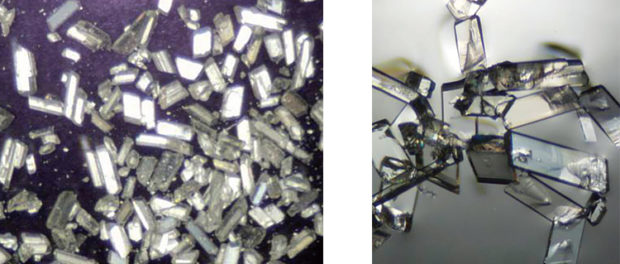
To combat this problem, Williams and his fellow researchers worked to synthesize a family of compounds based on simple guanidines designed to bind to contaminants and form insoluble crystals that are easily separated from water. They had difficulties getting a crystal structure of guanidine bound to nitrate, but when researchers left the guanidine solution out in the open air, a different type of crystal started to form.
The team analyzed them and discovered they contained carbonate, which forms when carbon dioxide from the air reacts with an alkaline solution.
Even though carbon capture is not a new tool for scientists looking for ways to combat global warming, the process Williams and researchers at ORNL developed requires significantly less energy to capture and later release the CO2 for storage.
“Current processes, while dirt cheap, need a lot of energy – up to 840 degrees Celsius – to release the CO2 gas,” Williams says. “The beauty of our process is that it only needs to be heated to 120 degrees.”
While not ready for market yet, the team is hopeful for prospects of future applications.
“We are working to design a simple system so that anyone could set it up and capture carbon right in their garage,” Williams says.
The UT-ORNL partnership is what prepared him for a career in chemistry, according to Williams.
“The lab is well-known both nationally and internationally and the fact that a graduate student can put ORNL on their resume is great,” Williams says. “We do ground-breaking research that has an impact; it’s something that can change the world.”
Originally published in Higher Ground, April 20, 2017.
Leave a comment